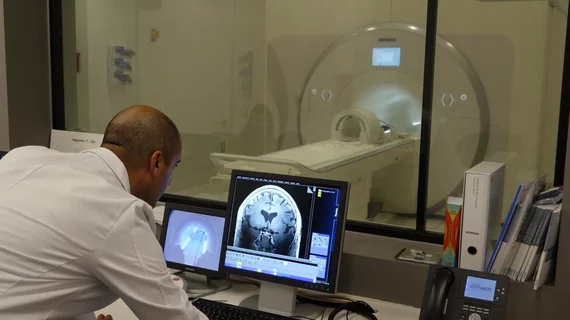
The 7T MRI scanner at the Keck School of Medicine of USC in Los Angeles has received FDA approval for clinical use. The school says this makes it the first of its kind in North America.
The scanner, installed at the school’s USC Mark and Mary Stevens Neuroimaging and Informatics Institute (INI) in February 2017, has already been used by researchers to examine brain activity, “unlocking a universe of detail never before seen.” The scanner was also used to produce an ultra-high-resolution scan of a Cushing’s disease patient, which was used for research published in the Journal of Neurosurgery.
“This device, which has already made its mark as a powerful tool to advance research in the neurosciences, is now accessible to clinical populations in addition to researchers,” Arthur W. Toga, PhD, a provost professor at the school and director of the INI, said in a prepared statement. “Clinicians across the university and beyond can now leverage all the benefits of increased spatial resolution to serve patients in need.”
“It's really a dramatic improvement,” Danny JJ Wang, PhD, director of imaging technology innovation at the INI and professor of neurology and radiology at the Keck School, said in the same statement. “Ideally, we want to look at the smallest group of neurons possible so we can start to pinpoint what's happening at the cellular level.”